

- Chromate chromium charge for free#
- Chromate chromium charge pdf#
- Chromate chromium charge download#
They belong to first inner transition series.
Lanthanoids: The 14 elements immediately following lanthanum, i.e., Cerium (58) to Lutetium (71) are called lanthanoids. The f-Block elements: The elements constituting the f -block are those in which the 4 f and 5 f orbitals are progressively filled in the latter two long periods. Zinc, cadmium, mercury are not regarded as transition metals due to completely filled d – orbital. 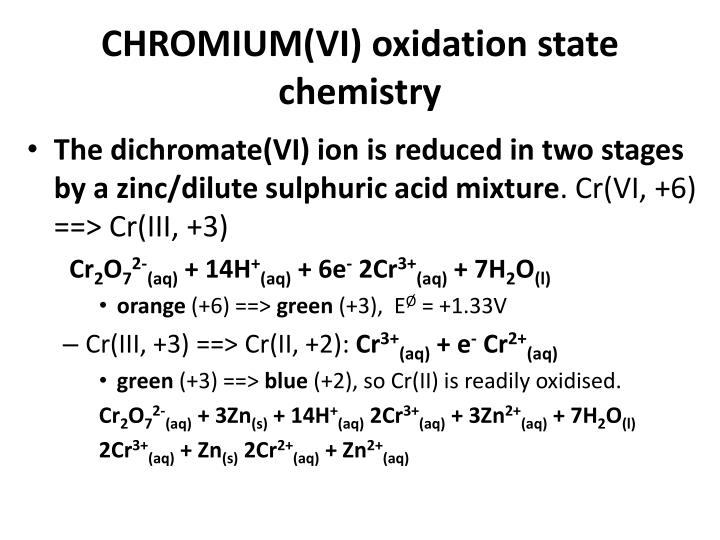
A transition element is defined as the one which has incompletely filled d orbitals in its ground state or in any one of its oxidation states.Their general electronic configuration is where (n – 1) stands for penultimate (last but one) shell.The elements lying in the middle of periodic table belonging to groups 3 to 12 are known as d – block elements.

The D and F-Block Elements Class 12 Notes Chemistry Revision notes in exam days is one of the best tips recommended by teachers during exam days. The revision notes help you revise the whole chapter 8 in minutes. These are the The d- and f- Block Elements class 12 Notes prepared by team of expert teachers.
Chromate chromium charge download#
Download revision notes for The d- and f- Block Elements class 12 Notes and score high in exams.
Chromate chromium charge pdf#
12 Chemistry notes Chapter 8 The d- and f- Block Elementsĭownload CBSE class 12th revision notes for chapter 8 The d- and f- Block Elements in PDF format for free. Users can download CBSE guide quick revision notes from m圜BSEguide mobile app and my CBSE guide website. It includes all the topics given in NCERT class 12 Chemistry text book. CBSE Guide The d- and f- Block Elements class 12 Notes ChemistryĬBSE guide notes are the comprehensive notes which covers the latest syllabus of CBSE and NCERT. Class 12 Chemistry notes on chapter 8 The d- and f- Block Elements are also available for download in CBSE Guide website. The best app for CBSE students now provides The d- and f- Block Elements class 12 Notes latest chapter wise notes for quick preparation of CBSE board exams and school based annual examinations.
Chromate chromium charge for free#
CBSE Class-12 Revision Notes and Key PointsĬBSE class 12 The d- and f- Block Elements class 12 Notes Chemistry in PDF are available for free download in m圜BSEguide mobile app. The d- and f- Block Elements class 12 Notes Chemistry. 12 Chemistry notes Chapter 8 The d- and f- Block Elements. CBSE Guide The d- and f- Block Elements class 12 Notes Chemistry. The color of these ions is pH dependent, as indicated by the color changes when the above reactions take place. If a shift in pH causes the solution to become more acidic (i.e. The equilibrium equation can be represented byīy Le Chatelier’s Principle, if certain conditions (concentration, temperature, pressure, volume, etc.) are changed, the amount of each ion present in solution is affected. I will try to take a different approach to your question (in terms of acidity and basicity).Ĭhromate (yellow) and dichromate (orange) ion are at equilibrium in solution. The complementary color of blue is red slash orange, and that is in fact the color we see in the dichromate ion!Īt the heart of all this is the principle that the colors we see are those wavelengths of light which on average are not absorbed by a large number (on the order of Avogadro's number) of molecules.Īn approach like this will only be reliable for very similar molecules like the two we have here. That means, if one of the bonds in the chromate ion, and thus two of the bonds in the dichromate ion, were absorbing a longer wavelength like we said earlier, on average we would expect something just longer than purple-ish, like blue, to be absorbed. Thus, in the case of the chromate ion, we see yellow, and across from yellow is the purple-ish region. Now to the color wheel! It is a general chemistry (often unexplained) fact that the color we see is the complementary color of the wavelength of a bond's vibration. This means that bond will vibrate at a lower frequency, and because frequency and wavelength are inversely related, that bond will absorb a longer wavelength of light. If you look at the structure of the chromate and dichromate ions next to each other (see here for structures: ), the only major difference between the two is that the Cr-O bond joining the two chromate ions (missing an oxygen) is now a single bond. I'm really excited for this because I get to reference the almighty color wheel!! Fair warning, this answer is much more qualitative than quantitative, but that's more interesting sometimes anyways.







 0 kommentar(er)
0 kommentar(er)
